El papel del nitrógeno en la agricultura y la contaminación por nitrato
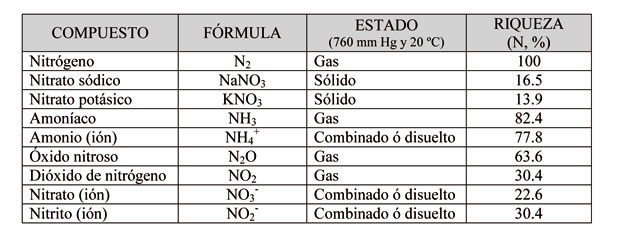
Las reacciones minerales en las que participa el nitrógeno
El nitrógeno (N) es un elemento esencial para los vegetales y junto con el fósforo (P) y el potasio (K) constituyen los tres macronutrientes (NPK) más importantes en la nutrición vegetal. Al mismo tiempo, como consecuencia de la actividad agrícola y ganadera, también participa en un conjunto de reacción que pueden afectar al medio ambiente y/o a la salud de las personas. Estas reacciones son las siguientes:
- El monóxido de nitrógeno (NO) se forma a partir del nitrógeno molecular (N2) cuando reacciona con el oxígeno molecular (O2):
N2 + O2 > 2 NO
- En el aire, el monóxido de nitrógeno (NO) se oxida con bastante facilidad dando dióxido de nitrógeno (NO2). Los gases NO y NO2, habitualmente reciben el nombre de óxidos de nitrógeno (NOx):
2 NO + O2 > 2 NO2
- El ión amonio (NH4+) aparece como consecuencia de la reacción ácido-base:
NH3 + H+ <> NH4+
- Mediante la 'síntesis de Haber', el nitrógeno del aire (N2) reacciona con el hidrógeno (H2) a alta presión y temperatura para dar amoníaco (NH3):
N2 + 3 H2 <> 2 NH3
- El amoníaco (NH3) se oxida a nitrito (NO2-):
2 NH3 + 3 O2 > 2 NO2- + 2 H+ + 2 H2O
- El ión amonio (NH4+) también se oxida a nitrito (NO2-):
2 NH4+ + 2 OH- + 3 O2 <> 2 H+ + 2 NO2- + 4 H2O
- El ión nitrito (NO2-) se oxida a nitrato (NO3-):
2 NO2- + O2 > 2 NO3-
El ciclo del nitrógeno en la naturaleza
El ciclo del N (Figura 1) tiene varias etapas y en cuatro de ellas participan las bacterias:
· Fijación: Consiste en la incorporación del N atmosférico a las plantas, por medio de la enzima nitrogenasa presente en algunas bacterias y cianobacterias que habitan en el suelo y en ambientes acuáticos, en ausencia de oxígeno. Para ello, los microorganismos (Rhizobium sp.) que llevan a cabo esta transformación (N2 > NH3 > NO3) viven en el interior de nódulos, siendo las leguminosas las especies huésped en las que suelen habitar y la leg-hemoglobina el pigmento rojo que caracteriza su actividad. Según algunos autores, la cantidad de N fijado por estas bacterias en la biosfera es del orden de 200 millones de toneladas anuales.
· Nitrificación o mineralización: Las raíces de las plantas que cultivamos tan solo pueden absorber dos formas de N: Nitrato (NO3-) y amonio (NH4+). El amonio se convierte en nitrato por medio de la nitrificación. La transformación del amonio en nitrato aumenta con la temperatura (> 10 °C) y el pH (5.5 – 6.5) del medio. El proceso se realiza en dos etapas: a) transformación del amonio (NH4+) en nitrito (NO2-) por medio de bacterias presentes en el suelo (nitrosomonas y nitrococcus); y b) transformación del nitrito (NO2-) en nitrato (NO3-) por medio de otras bacterias también presentes en el suelo (Nitrobacter). En realidad se trata de dos reacciones de tipo oxidativo, de las cuales las bacterias obtienen energía.
· Asimilación: Tiene lugar cuando las plantas absorben nitrato (NO3-) o amonio (NH4+) a través de sus raíces. En el interior de la planta, estas moléculas son metabolizadas y debidamente combinadas con azúcares y otras moléculas procedentes de la actividad fotosintética, el N se incorpora finalmente a aminoácidos, proteínas y ácidos nucleicos (ADN y ARN) u otras sustancias propias de cada especie. Los animales consumen estas sustancias y las transforman en otras similares de naturaleza animal.
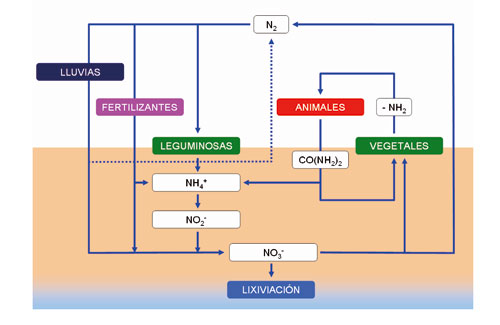
· Amonificación: Los compuestos nitrogenados (proteínas y ácidos nucleicos entre otros) ya sean de origen vegetal o animal, al igual que otros desechos que contienen nitrógeno, como la urea procedente de la orina, el ácido úrico procedente de las aves o los organismos muertos, se descomponen por bacterias presentes en el medio, liberando N, en forma de amonio –de ahí el nombre– previo paso por compuestos aminados (proteínas, peptonas y finalmente aminoácidos).
· Inmovilización: Es el proceso contrario a la mineralización, por medio del cual las formas inorgánicas (NH4+ y NO3-) son convertidas a N orgánico no asimilable.
· Desnitrificación. La reducción del nitrato (NO3-) a N2 y amonio (NH4+) a amoniaco recibe el nombre de desnitrificación. Se realiza por medio de las bacterias desnitrificadoras que revierten la acción de las bacterias fijadoras de N, con lo cual el N es devuelto a la atmósfera en forma de gas (N2). Este proceso ocasiona una pérdida de nitrógeno para el ecosistema; se da donde se acumula materia orgánica en condiciones anaeróbicas y alto pH. En condiciones de mucha humedad en el suelo, la falta de oxígeno obliga a ciertos microorganismos a emplear nitrato en vez de oxígeno para respirar (obtener energía).
· Aportaciones por lluvia: La lluvia aporta cantidades variables de N en forma de amonio, nitrato y óxidos de nitrógeno, lo que constituye una fuente importante de N para algunos ecosistemas naturales. Este aporte oscila entre 5 y 15 kg de N por hectárea y año. No obstante, para muchos sistemas agrícolas, este valor resulta insuficiente si lo comparamos con las necesidades que habitualmente se cubren con fertilizantes químicos.
La producción agrícola y el nitrógeno
La dosis óptima de nitrógeno (Fig. 2) que debemos aportar a un cultivo depende de tres factores: a) el cultivo; b) la “fertilidad” de la parcela en el momento de realizar la aplicación; y c) el objetivo que deseamos alcanzar. Por lo tanto, en la mayor parte de los casos, la decisión de utilizar una determinada dosis no puede tomarse a partir del mero cálculo de las extracciones que lleva a cabo el cultivo, tal como se ha venido haciendo durante muchos años, siguiendo las recomendaciones de los tratados clásicos de fertilización en agricultura.
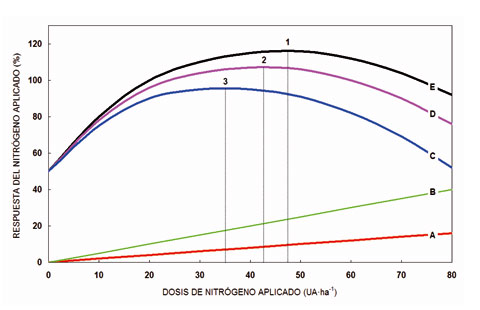
Las recomendaciones agronómicas sobre fertilización están basadas en trabajos experimentales y son válidas para cultivos y zonas concretas. Cuando comparamos (Fig. 2) el aumento de producción que experimenta un cultivo en función de la cantidad de nitrógeno aplicada por hectárea (UA, unidades arbitrarias), nos encontramos que la producción bruta (E) deja de crecer a partir de un determinado punto (1); se trata del óptimo agronómico. Si observamos la función de producción (D) que se obtiene cuando restamos el coste del N aplicado (A) a la producción bruta (E), obtenemos otro óptimo; se trata del óptimo económico (2) y finalmente, si fuéramos capaces de estimar el coste ambiental que representa la aplicación de N (B), restándolo de la producción bruta (E) obtendríamos otro óptimo; en este caso, se trataría del óptimo ambiental (3). De lo anteriormente expuesto se desprenden dos conclusiones: a) Que la aplicación óptima de N depende claramente del objetivo que deseamos alcanzar (agronómico, económico o ambiental); y b) que los tres óptimos están íntimamente relacionados entre sí (N ambiental < N económico < N agronómico).
La gestión del nitrógeno en una explotación agraria
En la ‘Información Técnica 147’, elaborada por D. Manuel Gil Martínez y publicada en 2004 por el Departamento de Agricultura y Alimentación del Gobierno de Aragón, aparecen enumerados los aspectos de gestión y control del N que deben ser considerados en una explotación agraria:
· Gestión individualizada: Por explotación y parcelas edafológicas homogéneas.
· Gestión de secano y regadío independiente: En regadío se considerará la forma de riego para calcular la dosis y la frecuencia de abonado.
· Variables de gestión: Se debe considerar la cantidad y la frecuencia de N aplicado (mineral y orgánico) y su capacidad de liberación.
· Materia orgánica del suelo: Es una variable de control periódico imprescindible.
· Nitrógeno disuelto en el agua de riego: Puede ser relevante para parcelas de regadío, especialmente en finales de cuenca.
· Restos de cosecha y cubiertas vegetales: Regeneran la materia orgánica del suelo y reducen el movimiento incontrolado del N en el suelo.
· Leguminosas: Hay que considerar el N que fijan y la aportación que realizan por medio de la materia orgánica que permanece en el suelo.
· Mineralización: La mineralización del N orgánico del suelo depende de la materia orgánica aportada y una constante agroambiental.
· Explotaciones ganaderas: La cantidad de materia orgánica depende del censo medio de las reses y su estado de desarrollo.
· Deyecciones ganaderas: La contaminación por deyecciones ganaderas depende de la localización y del sistema de explotación.
· Normativa: La normativa legal propia de cada comunidad debe ser conocida y respetada.
· Buenas prácticas: Las buenas prácticas agrícolas o ganaderas deben ser aplicadas en todo momento.
Orientaciones sobre abonado nitrogenado
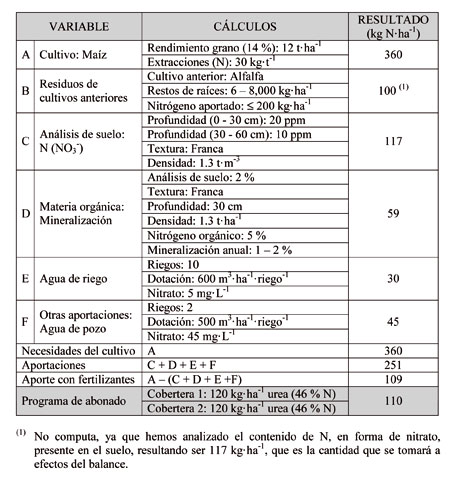
De acuerdo con la normativa vigente en materia de abonado nitrogenado, especialmente en las denominadas Zonas Vulnerables al Nitrógeno (ZVN), antes de tomar la decisión de aplicar una determinada cantidad de N a un cultivo debemos realizar un balance. Se trata de calcular las necesidades que presenta el cultivo, en función de la producción esperada y restar todas las aportaciones que van a ser realizadas, como consecuencia de: a) el cultivo anterior; b) la cantidad de estiércol aplicada; c) la materia orgánica que será mineralizada; d, los restos vegetales que puedan ser incorporados; e) el agua de riego; y f) otras aportaciones.
En la Tabla 2 presentamos un esquema del balance que podría utilizarse para este fin. Para elaborar correctamente un programa de abonado para un cultivo, además de lo anteriormente expuesto acorde con la normativa vigente, es necesario atender algunas recomendaciones prácticas.
Cómo cuantificar el N en la propia explotación
Desde hace varios años, en el mercado nacional existen equipos compactos que permiten conocer con suficiente precisión y fiabilidad la concentración de nitrógeno (N) o nitrato (NO3-) que tenemos en el suelo, en el agua de riego o incluso en el cultivo. En este artículo abordamos el primer uno de estos aspectos: la medida de la concentración de N en el suelo. Los resultados que mostramos (Tabla 3) proceden de las mediciones comparativas que, durante varios meses y en diferentes tipos de suelos, hemos realizado en el Departamento de Producción Vegetal (Fitotecnia), de la Escuela Técnica Superior de Ingenieros Agrónomos, de la Universidad Politécnica de Madrid (UPM), utilizando para ello un analizador de Hanna Instruments.
La toma de muestras es fundamental para que la interpretación de resultados sea correcta; de ahí la necesidad de extremar las precauciones a la hora de tomar la muestra de suelo.
- Toma de muestras: Para la determinación de la concentración de N en el suelo hay que partir de muestras que sean representativas de la parcela. Para ello, conviene recordar que la concentración de N varía con los horizontes naturales del suelo o con las diferentes capas que, como consecuencia del laboreo u otras prácticas culturales, se hayan podido establecer. Por lo tanto, suele ser razonable tomar muestras al menos a dos profundidades diferentes: entre 0 y 20 - 30 cm (Muestra A) y por debajo de 20 - 30 cm (Muestra B). En el caso de que se trate de parcelas no homogéneas, se recomienda dividir la parcela en tantas subparcelas como zonas diferentes puedan ser identificadas. Para ello, la parcela debe ser recorrida en zig-zag, a lo largo y ancho de toda su superficie, tomando pequeñas fracciones de las Muestras A y B, colocándolas en recipientes separados, hasta conseguir 1 kg de muestra, aproximadamente, para cada una de ellas.
- Preparación del extracto de saturación. En un recipiente de 500 ml se añaden 200 g de la muestra de suelo que queremos analizar. Lentamente y amasando con una espátula se va añadiendo agua destilada hasta alcanzar el punto de saturación, que aparece cuando la pasta brilla y fluye ligeramente cuando se inclina el recipiente. Una vez alcanzado el punto de saturación se anotan los ml de agua destilada utilizados en la operación de saturación. La muestra se deja en reposo durante una hora y se verifica el estado de saturación, añadiendo más agua destilada si fuera necesario.
La pasta saturada se transfiere con la misma espátula que ha sido utilizada para su elaboración, a un embudo Buchner, con papel de filtro ligeramente humedecido en su base y se aplica vacío. El filtrado, que debe salir casi incoloro, se recoge en el recipiente de cristal denominado kitasato y constituye el extracto de saturación. Para evitar la formación de algún precipitado a base de carbonato cálcico, se puede añadir una gota de una disolución de hexametafosfato sódico (1 g·100 ml-1) por cada 25 ml de extracto.
- Medida de la concentración de nitrato. Con un fotómetro multiparamétrico Hanna Instruments (HI 83214), la medida de la concentración de nitrato (NO3-) se puede realizar directamente sobre la muestra si se encuentra entre 0 y 30 mg·l-1. En el caso que sea superior, la muestra debe ser diluida con agua destilada y el resultado final que muestra la pantalla del equipo debe ser multiplicado por el factor de dilución utilizado; por ejemplo, si a 25 ml de extracto le añadimos 75 ml de agua destilada, hasta alcanzar un volumen final de 100 ml de disolución, el factor de dilución es 4.
El fotómetro multiparamétrico HI 83214 trabaja con una lámpara de tungsteno y un filtro de interferencia que deja pasar la radiación electromagnética de 420 nm. Cuando la muestra contiene nitrato, la adición de los reactivos que incorpora el método genera un color amarillo característico cuya intensidad es proporcional a la cantidad de nitrato que contiene la muestra. La resolución de la medida que obtenemos es de 0.1 mg·l-1. Al igual que ocurre con otros métodos, la presencia de iones superiores a las concentraciones que seguidamente se indican pueden producir interferencias: Bario (Ba+2), 1 mg·l-1; cloruro (Cl-), 1000 mg·l-1 y nitrito (NO2-), 50mg·l-1.

Resultados y conclusiones
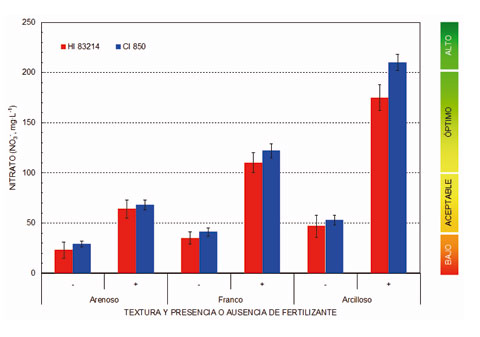
La Fig. 3 muestra de forma comparativa los resultados alcanzados al comparar la concentración de nitrato que presentan los extractos de saturación de seis muestras de suelos, caracterizadas por su textura y adición previa de fertilizantes nitrogenados. Los resultados obtenidos con el fotómetro multiparamétrico Hanna Instruments HI 83214 han sido comparados con los que se obtienen cuando las mismas muestras son analizadas mediante cromatografía iónica (MetronMH, mod. IC 850) a partir de patrones correctamente calibrados. Los resultados obtenidos permiten concluir que:
- Cuando se siguen de forma cuidadosa las instrucciones de preparación de muestras, los resultados que se alcanzan al medir la concentración de nitrato (NO3-) en extractos de saturación obtenidos a partir de muestras de suelo, con el espectrofotómetro multiparamétrico Hanna Instruments HI 83214, son comparables con los que se obtienen al analizar las muestras en un laboratorio especializado, mediante cromatografía iónica, a partir de patrones bien calibrados.
- Las diferencias que se obtienen se pueden explicar por el hecho de que el método que utiliza el equipo HI 83214 es ‘technique sensitive’, es decir, la forma de agitar el vial que contiene la muestra con los reactivos (número de agitaciones y frecuencia) debe ser realizada siempre de la misma forma y, a ser posible, por el mismo operador. La presencia de cloruro (Cl-) en el extracto de saturación, con una concentración superior a 1000 mg·l-1, también puede causar interferencias.
- Con independencia de las restantes aplicaciones que puede llevar a cabo, el espectrofotómetro multiparamétrico Hanna Instruments HI 83214 resulta ser un instrumento muy recomendable para la determinación del contenido en nitrato (NO3-) en parcelas de cultivo, especialmente dentro de las denominadas zonas vulnerables al nitrógeno.